Abstract
Background. Treatment of multiple myeloma (MM) has improved with novel therapies emerged in the last decade. However, patients refractory to treatment with standard drug classes including proteasome inhibitors, immunomodulatory drugs, and anti-CD38 monoclonal antibodies (RRMM patients) still constitute an unmet medical need, with median survival ranges no longer than 9 months (Richardson et al, 2007). Belantamab Mafodotin (Belamaf) is a novel anti-BCMA antibody conjugated to microtubule-disrupting agent monomethyl auristatin F. Belamaf showed anti-myeloma activity with an acceptable safety profile in RRMM patients in phase 1 (Trudel et al, 2019) and phase 2 trials (Lonial et al, 2020 & 2021). Belamaf is the first antibody drug conjugate approved in RRMM patients and real-life evaluations are needed. We aimed to assess the efficacy and safety of Belamaf administered as monotherapy in a cohort of Spanish RRMM patients.
Methods. An observational, retrospective and multicenter study was performed. RRMM patients who received at least one dose of Belamaf within compassionate use or expanded access programs in Spain between November 2019 and June 2021 were included. Researchers entered medical records in case report forms distributed to the participating sites. The primary endpoint was the overall response rate (ORR). Secondary endpoints were: overall survival (OS); duration of response (DoR); progression free survival (PFS); ocular and non-ocular treatment emergent adverse events (TEAEs) of any grade whose incidence was ≥15%, and those of grade ≥3 whose incidence was ≥5%.
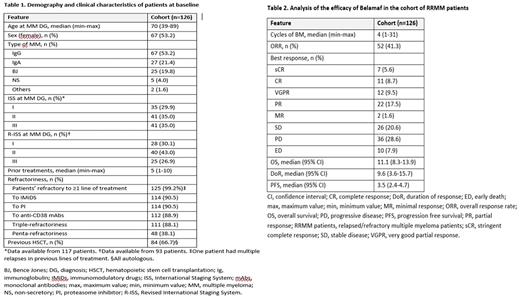
Results. One hundred and twenty-six patients from 59 hospitals, 67 of whom (53.2%) were women, were recruited. At MM diagnosis, median (range) age was 70 (39-89) years, and 27% of patients had R-ISS III. At study entry, 88% of patients were triple-class refractory. The median number of prior therapy lines was 5 (range, 1-10), and 67% of patients had received an autologous hematopoietic stem cell transplantation (Table 1). The median follow-up was 13 months. ORR was achieved in 52 (41.3%) patients. Thirty (23.8%) patients achieved at least very good partial response, and 11 (8.7%) and 7 (5.6%) patients showed complete response and stringent complete response, respectively. DoR and PFS were 9.6 (3.6-15.7) and 3.5 (2.4-4.7) months, respectively [median (95% CI)] Median OS was 11.1 months (8.3-13.9 months]) (Table 2).
Disease progression was the most common reason for treatment discontinuation (69 patients), while 5 (5%) patients discontinued Belamaf due to adverse events (1 due to the keratopathy). Seventy (55.6%) patients had at least one ocular TEAE. Keratopathy, which was reported in 64 (50.8%) patients, was the most commonly found ocular TEAE. Twenty-five (19.8%) of these cases were of grade ≥3. The most common ocular symptoms were blurry vision and dry eye, in 28 (22.2%) and 26 (20.6%) cases, respectively. One hundred and fifteen non-ocular TEAEs were documented in 56 (44.4%) patients, 45 (39.1%) grade ≥3, and were reported in 30 (23.8%) patients. Thrombocytopenia was the only hematologic TEAE whose incidence met the criteria specified in Methods. Thrombocytopenia occurred in 18 (14.3%) patients, being of grade ≥3 in 13 (10.3%) cases. Finally, infection, documented in 18 (14.3%) patients, was the most commonly found non-hematologic toxicity. Half of infection cases were of grade ≥3.
Conclusion. Belamaf showed a noticeable anti-myeloma activity, in this real-life series of RRMM patients, in the line with what has been observed in the DREAMM-2 clinical trial, when administered to a cohort of heavily pretreated RRMM patients in the context of either compassionate use or expanded access programs. The safety profile was manageable and consistent with previous studies.
Disclosures
De La Rubia:AMGEN, BMS, GSK, Janssen, Sanofi, Takeda: Consultancy. Alonso:Amgen: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; GSK: Honoraria; Janssen: Honoraria. Escalante:Sanofi: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees. Rios:Becton-Dickinson, Celgene, GSK, Janssen, Sanofi, Binding Site: Consultancy. Sampol:GSK: Consultancy. Gonzalez-Calle:Janssen: Consultancy; Janssen, Pfizer, Bristol-Myers Squibb/Celgene, GlaxoSmithKline: Honoraria, Speakers Bureau. Alegre:Janssen, BMS-Celgene, Amgen, Sanofi,GSK, Grifols, Pfizer, Abbvie, Novartis, Oncopeptide, Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal